Chapter 4 Indicator Tracking
Indicators are defined based on the GFMU reporting needs. However, countries may request for additional metrics, including country-level disaggregations based on the D2A framework. The indicators are aligned to global fund indicator matrix where modules group them and are classified by indicator codes. Each metric has a description which provides additional information on the analysis and interpretations.
Generally the GFMU tracks indicators in three main categories; baseline, targets and Achievement values as discussed below.
- Baseline - Provides the base measurement of the performance indicator before the implementation of the project activities. Its usually at the start of the reporting period.
- Targets - A specific, planned level of result to be achieved within an explicit timeframe. Usually at the end of the reporting period. Targets are estimated based on the the Global Fund indicator classes.
- Achievement - The actual measurement achieved in the reporting period. Usually at the end of the reporting period.
Below is an illustration of the indicator set up in Global Fund eTracker.
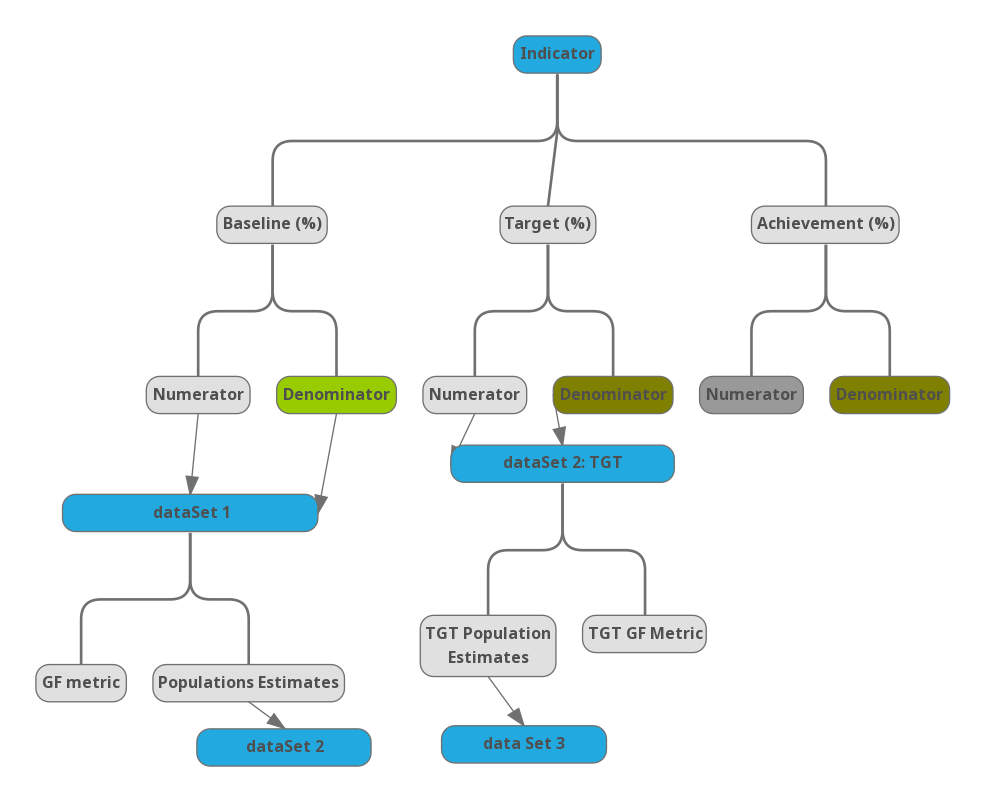
Figure 4.1: Global Fund eTracker Indicator Analysis
4.1 Indicators
| Code | Name of the Indicator | Description |
|---|---|---|
| HIV I-6 | GF - Estimated child HIV infections |
DescriptionThis indicator is to assess the impact of antiretrovial medicine programmes on the number of children acquiring HIV by estimating the HIV transmission rate from women living with HIV to their children. The transmission can be calculated by using the Spectrum model. In countries where data are available, facility attendance is high, and confirmatory tests are conducted systematically, efforts should be made to monitor the impact through directly assessing the percentage of children found to be HIV-positive among those born to HIV-positive mothers. All countries should make efforts to monitor the HIV status and survival of children born to HIV-positive women, gathered during follow-up health care visits. For further information refer to WHO publications on HIV monitoring and evaluation Numerator:Estimated children newly infected with HIV from mother-to-child transmission among children born in the previous 12 months to women living with HIV DenominatorEstimated children delivered by women living with HIV who delivered in the previous 12 months SourceModelled (SPECTRUM MODEL) Target typeNot applicable Disaggregation of reported resultsNot applicable ReferencesSame as in GARPR 2016 (Indicator 3.3) Worded differently in:
|
| HIV O-1(M) | GF - HIV+ve adults and children on treatment |
DescriptionThe reporting period is defined as any continuous 12-month period that has ended within a predefined number of months from the submission of the report. National reporting requirements can determine the predefined number of months. If the reporting period is 1 January to 31 December 2016, countries will calculate this indicator by using everyone who started antiretroviral therapy any time between 1 January and 31 December 2015. Retention at 12 months after starting antiretroviral therapy is defined as the outcome. Those who have died since starting therapy, those who have stopped therapy and those lost to follow-up as of month 12 (or 24, 36, 48, 60, etc.) are included in the denominator but not in the numerator. For example, people who started antiretroviral therapy between 1 January and 31 December 2014 will have reached their 12-month outcomes for the reporting period 1 January to 31 December 2015. As patients start antiretroviral therapy, monthly cohort data should be collected continuously for these patients. Data for monthly cohorts that have completed at least 12 months of treatment should then be aggregated. At facility level, patients who have transferrred out will not be counted either in the numerator or denominator. Patients who have transferred in will be counted in both numerator and denominator. Survival over longer durations of treatment provide a better picture of the long-term effectiveness of ART. If this indicator is only produced in a sub-set of facilities, comment should be added on the source of information and whether the information is representative of all ART sites. NumeratorPLHIV currently receiving antiretroviral therapy DenominatorPLHIV and on antiretroviral therapy who have a suppressed viral load at 12 months (<1000 copies/ml) SourceProgram monitoring tools; ART registers and cohort and group analysis forms Target typeNot applicable Disaggregation of reported results
ReferencesWHO SI guide 2015-ART-5; page 133; Global AIDS Monitoring 2017- Indicator 1.3; Page 39; worded differently. Percentage of adults and children living with HIV known to be on antiretroviral therapy 12 months after starting. |
| TB O-1a | GF - TB notification rate (All forms) |
DescriptionIt refers to all forms of TB cases that are bacteriologically confirmed or clinically diagnosed with active TB by a clinician. It includes- new and relapse cases that are;
It does not include- retreatment cases such as;
NumeratorNotified cases of all forms of TB-(i.E. Bacteriologically confirmed + clinically diagnosed) includes new and relapse cases DenominatorTotal Population SourceTB register Target typeNot applicable Disaggregation of reported resultsNot applicable |
| TCP-2 (M) | GF - TB Tx success rate |
DescriptionWhere applicable, report separately for all forms of TB cases provided with treatment in prisons, or by a specific type of health care provider or the community. This indicator is also reported as an coverage/output indicator to facilitate performance-based funding at each Progress Update and Disbursement Request (PU/DR). For further details on all forms of TB cases included under this indicator please refer to comments above (cell P14) - below: It includes- new and relapse cases that are;
It does not include- retreatment cases such as;
NumeratorTB cases, all forms, bacteriologically confirmed plus clinically diagnosed, successfully treated (cured plus treatment completed) among all TB cases registered for treatment during, new and relapse cases DenominatorNotified cases of all forms of TB-(i.E. Bacteriologically confirmed + clinically diagnosed) includes new and relapse cases SourceNumerator:Number of all forms of TB cases who were successfully treated in each disaggregation cateogry Denominator:Number of all forms of TB cases registered for treatment in each disaggregation category Target typeNon cumulative (C) Disaggregation of reported results
ReferencesFor details refer to indicator TB O-2a. Disaggregated data should be reported based on routine reporting, for example, in countries with electronic reporting systems or in a sample of randomly selected districts or sites |
| TB O-4 (M) | GF - RR TB and MDR-TB Tx success rate |
DescriptionThe period of assessment is 12 calendar months, usually counted from January to end December, and referred to as an annual cohort. All patients registered and starting treatment during this period are included in the calculation. In sites testing with Xpert MTB/RIF® alone, the indicator can be modified to include also RR-TB cases started on a full MDR-TB treatment regimen. Only laboratory confirmed RR-TB, MDR-TB and XDR-TB cases are counted for cohort reporting of Final Outcomes. It is measured 24 months after the end of the period of assessment. This gives sufficient time for most patients to complete their treatment and for the final culture results to be issued and recorded. All data can be extracted from the Second-line TB treatment register. For example- Patients on a second-line drug regimen to be assessed are those who started on second-line drugs in the current calendar year minus three. Thus, if the current calendar year is 2017, the outcomes collated will be for the cohort started on second-line drugs in calendar year 2014. The report due date will be Q1 of 2018. NumeratorCases with RR-TB and/or MDR-TB began second-line treatment DenominatorTB cases with RR-TB and/or MDR-TB notified SourceNumerator:Number of bacteriologically-confirmed XDR-TB cases enrolled on second-line anti-TB treatment who are successfully treated Denominator: Number bacteriologically-confirmed XDR-TB cases enrolled on second-line anti-TB treatment Target typeNot applicable Disaggregation of reported resultsXDR TB |
| TB I-2 | GF - TB incidence rate |
DescriptionRefer to WHO TB report 2015 annex 1 for methods of measuring or estimating TB incidence in a given year. In countries where the performance of TB surveillance systems do not allow the use notifications as a proxy for incidence, series of case notification rates should be carefully analyzed; and short-term forecasts may be used for planning and budgeting. WHO recommends that all countries strengthen their surveillance systems until TB notifications are a direct measure (or close proxy) of TB incidence. WHO also recommends that countries periodically assess TB incidence (its absolute values and trends) using a standard framework and tool for analyzing and documenting the reliability and coverage of TB notification data. NumeratorNotified cases of all forms of TB-(i.E. Bacteriologically confirmed + clinically diagnosed) includes new and relapse cases DenominatorTotal Population SourceTB recording and reporting system or Global TB report Target typeNot applicable Disaggregation of reported resultsNot applicable ReferencesWorld Health Organization. Global taskforce on TB impact measurement |
| HIV O-4a (M) | GF - MSMs reporting condom use |
DescriptionIf data is available on another time period, include in the comments section. If there are concerns that the data are not based on a representative sample, the interpretation of the survey data should reflect these concerns. Where different sources of data exist, the best available estimate should be used. For further information refer to: Operational guidelines for monitoring and evaluation of HIV programmes for sex workers, men who have sex with men, and transgender people. Chapel Hill (NC): MEASURE Evaluation; 2011 NumeratorMSMs reported condom used the last time they had anal sex with a male partner DenominatorMSMs who reported having had anal sex with a male partner in the last six months SourceBehavioural surveillance (BSS) or other special survey Target typeNot applicable Disaggregation of reported results
ReferencesWHO SI guide 2015-PREV.1.b; page 75; Global AIDS Monitoring 2017- Indicator 3.6B; Page 68 |
| HIV O-5 (M) | GF - Sex workers reporting condom use |
DescriptionThis indicator asks about commercial sex in the past 12 months. If data is available on another time period, such as last three or 12 months, include in the comments section. If there are concerns that the data are not based on a representative sample, the interpretation of the survey data should reflect these concerns. Where different sources of data exist, the best available estimate should be used. NumeratorSex workers reported condom used with their last client DenominatorSex workers who reported having commercial sex in the last 12 months SourceBehavioural surveillance (BSS) or other special survey Target typeNot applicable Disaggregation of reported results
ReferencesFor further information refer to: Operational guidelines for monitoring and evaluation of HIV programmes for sex workers, men who have sex with men, and transgender people. Chapel Hill (NC): MEASURE Evaluation; 2011 WHO SI guide 2015-PREV.1.b; page 75; Global AIDS Monitoring 2017- Indicator 3.6A; Page 66 |
| KP-1a (M) | GF - MSMs with HIV prevention programs |
DescriptionThese indicators aim to monitor coverage of HIV prevention programs using program data and population size estimates. Where size estimations are not available, countries will be required to undertake estimation exercise as soon as possible. Until the revised estimates are provided, available estimates will be used as denominators. Data is generated by counting people who receive a defined package of services that includes the minimum specified components- BCC; provision of consumables (condoms; lubricants, needles and syringes as needed); referral to another service such as STI diagnosis and treatment, HIV testing and counseling, etc. In addition, it could include other interventions from the comprehensive package of services. The components of the package of HIV prevention interventions should be defined at country level and tailored to the needs of the target population. Refer to the comprehensive package of services recommended by technical partners- Tool to set and monitor targets for HIV prevention, diagnosis, treatment and care for key populations: supplement to the 2014 consolidated guidelines for HIV prevention, diagnosis, treatment and care for key populations. Geneva: World Health Organization; 2015 Data collection requires reliable tracking systems that are designed to count the number of individual “clients served” at the same service or across services as opposed to the “client visits”. This can be ensured through implementation of Unique Identification Codes (UIC). In the absence of UIC, report on the number of contacts until the time when a system to avoid double counting is set up. Agree on a timeframe for setting up such system and ensure adequate funds are available. The coverage data from routine reporting will be triangulated with the coverage from survey data for overall impact assessment. When targeting “other vulnerable populations” specify in the comments column of the performance framework which populations are being targeted. NumeratorMSMs reached with HIV prevention programs defined package of services DenominatorEstimated MSMs SourceNumerator: Program records Denominator: Estimated population size Target typeNon cumulative (A/ B/ E ) or Cumulative annually (D) Disaggregation of reported resultsNot applicable |
| KP-1a (M)b | GF - TGT MSMs with HIV prevention programs |
DescriptionThese indicators aim to monitor coverage of HIV prevention programs using program data and population size estimates. Where size estimations are not available, countries will be required to undertake estimation exercise as soon as possible. Until the revised estimates are provided, available estimates will be used as denominators. Data is generated by counting people who receive a defined package of services that includes the minimum specified components- BCC; provision of consumables (condoms; lubricants, needles and syringes as needed); referral to another service such as STI diagnosis and treatment, HIV testing and counseling, etc. In addition, it could include other interventions from the comprehensive package of services. The components of the package of HIV prevention interventions should be defined at country level and tailored to the needs of the target population. Refer to the comprehensive package of services recommended by technical partners- Tool to set and monitor targets for HIV prevention, diagnosis, treatment and care for key populations: supplement to the 2014 consolidated guidelines for HIV prevention, diagnosis, treatment and care for key populations. Geneva: World Health Organization; 2015 Data collection requires reliable tracking systems that are designed to count the number of individual “clients served” at the same service or across services as opposed to the “client visits”. This can be ensured through implementation of Unique Identification Codes (UIC). In the absence of UIC, report on the number of contacts until the time when a system to avoid double counting is set up. Agree on a timeframe for setting up such system and ensure adequate funds are available. The coverage data from routine reporting will be triangulated with the coverage from survey data for overall impact assessment. When targeting “other vulnerable populations” specify in the comments column of the performance framework which populations are being targeted. NumeratorTGT MSMs reached with HIV prevention programs defined package of services DenominatorTGT Estimated MSMs SourceNumerator: Program records Denominator: Estimated population size Target typeNon cumulative (A/ B/ E ) or Cumulative annually (D) Disaggregation of reported resultsNot applicable |
| KP-1c (M) | GF - Sex workers with HIV programs | |
| KP-1c (M)b | GF - TGT sex workers with HIV programs | |
| KP-3c (M) | GF - Sex workers with HIV result |
DescriptionCoverage will be assessed based on population size estimates. Where these are not available, countries will be required to undertake a size estimation as soon as possible. Until the revised estimates are provided, available, estimates will be used. Coverage data from routine reporting will be triangulated with the coverage from survey data for overall impact assessment. If data on persons who retest are not available, this indicator (reported as numbers only) will give information on the number of times HIV testing and counseling services were delivered, rather than the number of individuals who received HIV testing and counseling services. NumeratorSex workers received an HIV test and know their results DenominatorEstimated sex workers SourceNumerator: Program records Denominator:Estimated population size Target typeNon cumulative (A/ B/ E ) or Cumulative annually (D) Disaggregation of reported resultsNot applicable ReferencesWHO SI guide 2015: HTS 7; page 100 Global AIDS Monitoring 2017- Indicator 3.4; Page 62; Different indicator. Knowledge of HIV status among sex workers |
| KP-3c (M)b | GF - TGT Sex workers with HIV result |
DescriptionCoverage will be assessed based on population size estimates. Where these are not available, countries will be required to undertake a size estimation as soon as possible. Until the revised estimates are provided, available, estimates will be used. Coverage data from routine reporting will be triangulated with the coverage from survey data for overall impact assessment. If data on persons who retest are not available, this indicator (reported as numbers only) will give information on the number of times HIV testing and counseling services were delivered, rather than the number of individuals who received HIV testing and counseling services. NumeratorTGT Sex workers received an HIV test and know their results DenominatorTGT Estimated sex workers SourceNumerator: Program records Denominator:Estimated population size Target typeNon cumulative (A/ B/ E ) or Cumulative annually (D) Disaggregation of reported resultsNot applicable ReferencesWHO SI guide 2015: HTS 7; page 100 Global AIDS Monitoring 2017- Indicator 3.4; Page 62; Different indicator. Knowledge of HIV status among sex workers |
| Kp-1e | GF - Vulnerable population with HIV programs | |
| Kp-1eb | GF - TGT Vulnerable population with HIV programs | |
| KP-3e | GF - Vulnerable population with HIV results |
DescriptionCoverage will be assessed based on population size estimates. Where these are not available, countries will be required to undertake a size estimation as soon as possible. Until the revised estimates are provided, available, estimates will be used. Coverage data from routine reporting will be triangulated with the coverage from survey data for overall impact assessment. If data on persons who retest are not available, this indicator (reported as numbers only) will give information on the number of times HIV testing and counseling services were delivered, rather than the number of individuals who received HIV testing and counseling services. When targeting “other vulnerable populations” specify in the comments column of the performance framework which populations are being targeted. NumeratorOther vulnerable populations received an HIV test and know their results DenominatorEstimated other vulnerable populations SourceNumerator: Program records Denominator: Estimated population size Target typeNon cumulative (A/ B/ E ) or Cumulative annually (D) Disaggregation of reported resultsNot applicable ReferencesWHO SI guide 2015: HTS 7; page 100 |
| Kp-3eb | GF - TGT Vulnerable population with HIV results |
DescriptionCoverage will be assessed based on population size estimates. Where these are not available, countries will be required to undertake a size estimation as soon as possible. Until the revised estimates are provided, available, estimates will be used. Coverage data from routine reporting will be triangulated with the coverage from survey data for overall impact assessment. If data on persons who retest are not available, this indicator (reported as numbers only) will give information on the number of times HIV testing and counseling services were delivered, rather than the number of individuals who received HIV testing and counseling services. When targeting “other vulnerable populations” specify in the comments column of the performance framework which populations are being targeted. NumeratorTGT Other vulnerable populations received an HIV test and know their results DenominatorTGT Estimated other vulnerable populations SourceNumerator: Program records Denominator: Estimated population size Target typeNon cumulative (A/ B/ E ) or Cumulative annually (D) Disaggregation of reported resultsNot applicable ReferencesWHO SI guide 2015: HTS 7; page 100 |
| PMTCT-1 | GF - Pregnant Women with HIV results |
DescriptionCoverage will be assessed based on population size estimates. Population based survey data (for example, DHS or AIDS Indicator Survey), when available, will be used for triangulation purposes. This indicator enables countries to monitor trends over time in HIV testing among pregnant women. Disaggregated data by regions may allow to identify lower performing areas. While it is not feasible to avoid double counting entirely, countries should ensure data collection and reporting system is in place to miniumize it, such as using patient held and facility held ANC records to doucment that testing took place Include those with previously known HIV infection in the numerator- even if they do not receive an HIV test, their HIV infection is identified for susequent PMTCT interventions NumeratorPregnant women who know their HIV status DenominatorEstimated pregnant women who delivered within the past 12 months SourceNumerator: Program records, e.g. ANC registers, labour and delivery registers. Denominator: Estimates from central statistics office, UN Population Division or vital statistics. Target typeNon cumulative (B) or Cumulative annually (D) Disaggregation of reported resultsNot applicable ReferencesWHO SI guide 2015: HTS.4 (page 99) and MTCT.1; Page 160 |
| PMTCT-1b | GF - TGT Pregnant Women with HIV results |
DescriptionCoverage will be assessed based on population size estimates. Population based survey data (for example, DHS or AIDS Indicator Survey), when available, will be used for triangulation purposes. This indicator enables countries to monitor trends over time in HIV testing among pregnant women. Disaggregated data by regions may allow to identify lower performing areas. While it is not feasible to avoid double counting entirely, countries should ensure data collection and reporting system is in place to miniumize it, such as using patient held and facility held ANC records to doucment that testing took place Include those with previously known HIV infection in the numerator- even if they do not receive an HIV test, their HIV infection is identified for susequent PMTCT interventions NumeratorTGT Pregnant women who know their HIV status DenominatorTGT Estimated pregnant women who delivered within the past 12 months SourceNumerator: Program records, e.g. ANC registers, labour and delivery registers. Denominator: Estimates from central statistics office, UN Population Division or vital statistics. Target typeNon cumulative (B) or Cumulative annually (D) Disaggregation of reported resultsNot applicable ReferencesWHO SI guide 2015: HTS.4 (page 99) and MTCT.1; Page 160 |
| PMTCT-2.1 | GF - HIV +ve pregnant women on ART |
DescriptionCountries are encouraged to track and report the number of women receiving the various regimens so that the impact of antiretroviral medicines on mother-to-child transmission can be modelled based on their efficacy. If countries do not have a system for collecting and reporting this data, they should establish one. Efforts should be made to remove women captured twice in the reporting systems. To ensure comparability, the Spectrum output is used for the denominator for global analysis. NumeratorHIV + pregnant women who received art during pregnancy DenominatorEstimated HIV positive pregnant women who delivered SourceNumerator: Program records, e.g. PMTCT registers, ARV registers. Denominator:Estimation models such as Spectrum or antenatal clinic surveillance surveys combined with demographic data and appropriate adjustments related to the coverage of antenatal clinic surveys. Target typeNon cumulative (B) or Cumulative annually (D) Disaggregation of reported resultsNot applicable ReferencesWHO SI guide 2015: MTCT.2; page 161 Global AIDS Monitoring 2017- Indicator 2.3; Page 51; worded differently. % of HIV positive pregnant women living with HIV who received anti-retroviral medicines to reduce the risk of mother to child transmission |
| PMTCT-2.1b | GF - TGT HIV +ve pregnant women on ART |
DescriptionCountries are encouraged to track and report the number of women receiving the various regimens so that the impact of antiretroviral medicines on mother-to-child transmission can be modelled based on their efficacy. If countries do not have a system for collecting and reporting this data, they should establish one. Efforts should be made to remove women captured twice in the reporting systems. To ensure comparability, the Spectrum output is used for the denominator for global analysis. NumeratorTGT HIV + pregnant women who received art during pregnancy DenominatorTGT Estimated HIV positive pregnant women who delivered SourceNumerator: Program records, e.g. PMTCT registers, ARV registers. Denominator:Estimation models such as Spectrum or antenatal clinic surveillance surveys combined with demographic data and appropriate adjustments related to the coverage of antenatal clinic surveys. Target typeNon cumulative (B) or Cumulative annually (D) Disaggregation of reported resultsNot applicable ReferencesWHO SI guide 2015: MTCT.2; page 161 Global AIDS Monitoring 2017- Indicator 2.3; Page 51; worded differently. % of HIV positive pregnant women living with HIV who received anti-retroviral medicines to reduce the risk of mother to child transmission |
| TCS-1 (M) | GF - PLHIV on ART |
DescriptionThe count should not include people who have stopped treatment, died or emigrated to another country or who are otherwise lost to follow-up at the facility during this period. Protocols should be in place to avoid duplicate counting of individuals across facilities or over time. This indicator does not include antiretroviral medicines taken only for preventing mother-to-child transmission and post-exposure prophylaxis. This indicator includes pregnant women living with HIV who are receiving lifelong antiretroviral therapy. Countries should triangulate the numerator from programme data with national procurement and drug monitoring systems and adjust reported numbers as appropriate. Countries that undertake data quality assessments or reviews that monitor the extent to which facilities are able to accurately report the number of people on treatment during reporting periods should also adjust programme numerator data to account for these inconsistencies. Estimates of coverage of antiretroviral therapy from surveys can also be used to inform or validate the numerator. Note that surveys that only capture self-reported data on treatment uptake should not be used, since self-reported data has been shown to be of limited quality. NumeratorPLHIV currently receiving antiretroviral therapy DenominatorEstimated PLHIV SourceNumerator: Program records, e.g. ART registers and corresponding cross-sectional reporting forms. Denominator: HIV estimation models such as Spectrum Target typeNon cumulative E Disaggregation of reported results
*To be reported from selected countries ●To be reported in cases where data for age groups 15-19 and 20-24 is not available ReferencesWHO SI guide 2015: ART.3; page 132 Global AIDS monitoring 2017- indicator 1.2; Page- 37; worded differently. Percentage and number of adults and children on antiretroviral therapy among all adults and children living with HIV at the end of the reporting period |
| TCS-1 (M)b | GF - TGT PLHIV on ART |
DescriptionThe count should not include people who have stopped treatment, died or emigrated to another country or who are otherwise lost to follow-up at the facility during this period. Protocols should be in place to avoid duplicate counting of individuals across facilities or over time. This indicator does not include antiretroviral medicines taken only for preventing mother-to-child transmission and post-exposure prophylaxis. This indicator includes pregnant women living with HIV who are receiving lifelong antiretroviral therapy. Countries should triangulate the numerator from programme data with national procurement and drug monitoring systems and adjust reported numbers as appropriate. Countries that undertake data quality assessments or reviews that monitor the extent to which facilities are able to accurately report the number of people on treatment during reporting periods should also adjust programme numerator data to account for these inconsistencies. Estimates of coverage of antiretroviral therapy from surveys can also be used to inform or validate the numerator. Note that surveys that only capture self-reported data on treatment uptake should not be used, since self-reported data has been shown to be of limited quality. NumeratorTGT PLHIV currently receiving antiretroviral therapy DenominatorTGT Estimated PLHIV SourceNumerator: Program records, e.g. ART registers and corresponding cross-sectional reporting forms. Denominator: HIV estimation models such as Spectrum Target typeNon cumulative E Disaggregation of reported results
*To be reported from selected countries ●To be reported in cases where data for age groups 15-19 and 20-24 is not available ReferencesWHO SI guide 2015: ART.3; page 132 Global AIDS monitoring 2017- indicator 1.2; Page- 37; worded differently. Percentage and number of adults and children on antiretroviral therapy among all adults and children living with HIV at the end of the reporting period |
| TCP-3 | GF - Labs with adq. performance of SMs |
DescriptionExternal quality assurance for smear microscopy is performed by rechecking slides. No error of any type is considered a target for optimal performance. Any major error (high false-positive or high false-negative) may indicate unacceptable performance. NumeratorLaboratories showing adequate performance in external quality assurance for smear microscopy among the total laboratories undertake smear microscopy DenominatorTotal laboratories undertaking smear microscopy SourceNumerator: NTP administration records Denominator: NTP administration records Target typeNon cumulative (B) Disaggregation of reported resultsNot applicable |
| TCP-5 | GF - Under 5 incontact with TB patients |
DescriptionNumerator:Children <5 in contact with TB patients who began isonizide preventive therapy DenominatorNot applicable SourceNumerator: Program records Denominator: Not applicable Target typeNon cumulative (A) Disaggregation of reported resultsNot applicable |
| TCP 6a | GF - TB cases (all forms) notifed to prisoners |
DescriptionNumeratorDenominatorSourceTB register at the basic management unit, community health unit Target typeNot applicable Disaggregation of reported resultsNot applicable |
| TB/HIV-3.1 | GF - PLHIV screened for TB |
DescriptionIndicator measurement same as before, wording revised by partners. Intensified TB case finding should be implemented at all HIV care and treatment facilities and TB status of people living with HIV should be assessed at every visit during the reporting period. It is also important to monitor implementation of the entire cascade of care, starting from symptom screening to diagnosis and treatment of TB. This necessitates close coordination between the NACP and NTP but responsibility of reporting lies with the NACP NumeratorPLHIV in care (including pmtct) who are screened for TB in HIV care or treatment settings DenominatorPLHIV currently receiving antiretroviral therapy SourceNumerator: pre-ART and ART register and cross-sectional quarterly reports Denominator: pre-ART and ART register and cross-sectional quarterly reports Target typeNon cumulative (C) Disaggregation of reported resultsNot applicable ReferencesWHO SI guide 2015: LINK.18; page 119 A guide to monitoring and evaluation for collaborative TB/HIV activities: 2015 revision- Indicator B.1; Page 22- |
| TB/HIV-6 (M) | GF - HIV+ve new and relapse TB patients on ART |
DescriptionPrompt TB treatment and early ART are critical for reducing the mortality due to HIV-associated TB and must be the highest-priority activity for both the NACP and NTP. While TB treatment should be started immediately, ART should be started within 8 weeks of TB diagnosis, given that all are eligible for ART irrespective of their CD4 cell count. Although it is important that ART status of all HIV positive TB patients is assessed, this indicator considers only new and relapse patients to avoid double counting. Cases with undocumented TB treatment history should be counted as new cases. TB and HIV programmes should aim to achieve TB treatment and ART in more than 90% of HIV positive TB patients. However, this indicator may miss patients diagnosed towards the end of reporting period whose ART treatment status may not be updated in the TB registers. Also, this indicator does not capture timeliness of ART initiation. NumeratorHIV + new and relapse TB patients on art during TB treatment DenominatorHIV + new and relapsed TB patients registered . SourceNumerator and Denominator: TB register at the basic management unit, Pre-ART register and ART register. Target typeNon cumulative (C) Disaggregation of reported resultsNot applicable ReferencesWHO SI 2015 guidelines: LINK.16; page 118 A guide to monitoring and evaluation for collaborative TB/HIV activities: 2015 revision- Indicator A.4; Page 18- |
| MDR-TB 2 (M) | GF - RR-TB and MDR-TB cases notified |
DescriptionTo be computed separately for patients detected with rifampicin resistant TB (RR-TB) alone in sites using Xpert MTB/RIF NumeratorTB cases with RR-TB and/or MDR-TB notified DenominatorNot applicable SourceNumerator: Lab register for culture, DST and xpert Denominator: Not applicable Target typeNon cumulative (A) Disaggregation of reported results
References’Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis: WHO, 2014. Annex 5, page 407; |
| MDR-TB 2 (M)b | GF - TGT RR-TB and MDR-TB cases notified |
DescriptionTo be computed separately for patients detected with rifampicin resistant TB (RR-TB) alone in sites using Xpert MTB/RIF NumeratorTGT TB cases with RR-TB and/or MDR-TB notified DenominatorNot applicable SourceNumerator: Lab register for culture, DST and xpert Denominator: Not applicable Target typeNon cumulative (A) Disaggregation of reported results
References’Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis: WHO, 2014. Annex 5, page 407; |
| MDR-TB 3 (M) | GF - RR-TB and MDR-TB cases on second-line Tx | Confirmed cases fully investigated & class. |
| MDR-TB 3 (M)b | GF - TGT RR-TB and MDR-TB cases on second-line Tx |
DescriptionThe programme manager is responsible to ensure that all patients in whom RR-TB or MDR-TB is detected are placed on appropriate treatment in the shortest time possible. This may also apply to patients at risk of infection with RR-TB but who are not confirmed (presumptive). Patients detected with rifampicin-resistant TB (RR-TB) in sites using Xpert MTB/RIF to be included in the denominator as well as numerator.
A comparison of enrolled to identified RR-TB/MDR-TB cases gives an indication of access to care although patients started on treatment may have been detected prior to the period of assessment. Comparator data are sourced from the Laboratory register for culture, Xpert MTB/RIF® and DST (using the date of DST result). The suggested period of assessment is six calendar months, the first usually counted from January to end June and July to end December. Indicators are measured in the month following the end of the six-month period. NumeratorTGT cases with RR-TB and/or MDR-TB began second-line treatment DenominatorNot applicable SourceNumerator: Second line TB treatment register Denominator: Not applicable Target typeNon cumulative (A) Disaggregation of reported results
|
| MDR-TB 4 | GF - RR-TB and MDR-TB cases on treatement lost to follow up |
DescriptionPatients with rifampicin- resistant TB (RR-TB) in sites using Xpert MTB/ RIF who are on treatment to be included in the denominator as well as numerator Lost to follow up refers to treatment interruption for two or more consecutive months for any reason without medical approval. Treatment for MDR-TB may take 9 months (short regimens) to 20 months or more and final outcomes can thus only be assessed one to three years after the start of enrolment. The programme manager often needs an indication of how patients are faring, well before that. This is particularly important when a drug-resistant TB treatment programme is starting. Once a program “matures,” the final outcomes become more useful to monitor. All lab confirmed RR-TB and MDR-TB patients registered and starting treatment during the period of assessment are counted for reporting of Interim Results. The denominator also includes XDR-TB cases started on prescribed treatment with second-line drugs The suggested period of assessment is six calendar months. This is usually counted from January to end June and July to end December. Indicators are measured three months after the end of the six-month period. There will be a six month timelag in reporting results from the end of a six monthly cohort. NumeratorCases with RR-TB and/or MDR-TB started on treatment for MDR-TB lost to follow up during the first six months of treatment DenominatorCases with RR-TB and/or MDR-TB began second-line treatment SourceNumerator: Second line TB treatment register Denominator: Second line TB treatment register Target typeNon cumulative (C) Disaggregation of reported results |
| M&E-1 | GF - Reporting units submitting timely reports |
DescriptionNumeratorHMIS or other routine reporting units submitting timely reports according to national guidelines DenominatorTotal HMIS or other routine reporting units SourceNumerator: HMIS, program records Denominator: HMIS, program records Target typeNon cumulative (B) Disaggregation of reported resultsReferencesHSS |
| TCS-3.1 | GF - PLHIV and on ART with suppressed VL |
DescriptionThe +/- 3 months allows to know if someone is still “on ART”. For example, for the cohort of people on ART in 2015, one would need to wait until March 2016 to know for sure if they are on ART because if they were supposed to come in December, but they are late and come within 3 months from their appointment, they are still counted to be on ART. But in March (3 months after their Dec appointment), if they do not come, then they are counted as not on ART in Dec 2015. In Dec 2015, one would know for sure about the cohort that started in Sept 2014. The +/- 3months period allows for accommodating reporting practices that may vary from country to country- some countries report based on the Sept cohort and others try to wait until March; and some others report end Dec for the full calendar cohort, and just make assumptions that everyone is on ART (no drop out for the Nov/Dec cohort you may not have information on yet) at the time of reporting. As an EWI of HIVDR, it reflects ability of facility to attain a level of care that avoids HIVDR. Good performance is >85%; passable performance is >70%. NumeratorPLHIV and on antiretroviral therapy who have a suppressed viral load at 12 months (<1000 copies/ml) DenominatorPLHIV who intiated art 12 months (±3 months) before the start of the reporting period SourceNumerator: Number of PLHIV who initiated ART 12 months (±3 months) before the start of the reporting period and who have a suppressed viral load (<1000 copies/ml) at 12 months after initiating ART in each disaggregation category Denominator: Number ofpeople living with HIV who intiated ART 12 months (±3 months) before the start of the reporting period in each disaggregation category. Target typeNot applicable Disaggregation of reported results |
| CM-1a(M) | GF - Suspected Malaria tested |
DescriptionFor countries that maintain a record of suspected cases, use the ‘number of suspected cases’ as denominator Where countries do not keep record of suspected cases, the following method used for World Malaria Report data, will be used to calculate suspected cases
In cases where reported cases are not differentiated as confirmed and presumed, the positive cases from lab register can be used a proxy for confirmed cases. The latter assumes that all those confirmed as malaria were treated. NumeratorSuspected malaria cases received a parasitological test DenominatorAll suspected malaria cases present SourceNumerator: Lab register or records of RDT use Denominator: Suspect register/ OPD register/ Treatment register Target typeNon cumulative (C) Disaggregation of reported results
|
| CM-1a(M)b | GF - TGT Suspected Malaria tested |
DescriptionFor countries that maintain a record of suspected cases, use the ‘number of suspected cases’ as denominator Where countries do not keep record of suspected cases, the following method used for World Malaria Report data, will be used to calculate suspected cases
In cases where reported cases are not differentiated as confirmed and presumed, the positive cases from lab register can be used a proxy for confirmed cases. The latter assumes that all those confirmed as malaria were treated. NumeratorTGT Suspected malaria cases received a parasitological test DenominatorTGT All suspected malaria cases present SourceNumerator: Lab register or records of RDT use Denominator: Suspect register/ OPD register/ Treatment register Target typeNon cumulative (C) Disaggregation of reported results
|
| CM-2a (M) | GF - Confirmed Malaria treated |
DescriptionPerformance assessment will be based on trends in malaria cases in the country NumeratorConfirmed malaria cases received first-line antimalarial treatment according to national policy DenominatorConfirmed malaria cases present SourceNumerator: OPD register/ Malaria treatment register/ Pharmacy Denominator: Lab registers Target typeNon cumulative (C) Disaggregation of reported results
|
| CM-2a (M)b | GF - TGT Confirmed Malaria treated |
DescriptionPerformance assessment will be based on trends in malaria cases in the country NumeratorTGT confirmed malaria cases received first-line antimalarial treatment according to national policy DenominatorTGT confirmed malaria cases present SourceNumerator: OPD register/ Malaria treatment register/ Pharmacy Denominator: Lab registers Target typeNon cumulative (C) Disaggregation of reported results
|
| CM-5 (M) | GF - Confirmed cases fully investigated and classifed |
DescriptionIncluding case investigation form, focus investigation form and active case detection. NumeratorConfirmed cases fully investigated and classified DenominatorConfirmed malaria cases present SourceMalaria case investigation database Target typeNon cumulative (C) Disaggregation of reported resultsReferencesDisease surveillance for malaria elimination- an operational manual, WHO, June 2013 |
| CM-5 (M)b | GF - TGT confirmed cases fully investigated and classifed |
DescriptionIncluding case investigation form, focus investigation form and active case detection. NumeratorTGT confirmed cases fully investigated and classified DenominatorTGT confirmed malaria cases present SourceMalaria case investigation database Target typeNon cumulative (C) Disaggregation of reported resultsReferencesDisease surveillance for malaria elimination- an operational manual, WHO, June 2013 |
| CM-6 (M) | GF - Malaria foci fully investigated and classified |
DescriptionMalaria focus investigation form completed, including data from an entomological investigation and registered (on register, with maps of each focus) (# & %) NumeratorMalaria foci fully investigated and classified DenominatorTGT confirmed malaria cases present SourceMalaria case investigation database Target typeNon cumulative (C) Disaggregation of reported resultsReferencesDisease surveillance for malaria elimination- an operational manual, WHO, June 2013 |
| CM-6 (M)b | GF - TGT Malaria foci fully investigated and classified |
DescriptionMalaria focus investigation form completed, including data from an entomological investigation and registered (on register, with maps of each focus) (# & %) NumeratorTGT malaria foci fully investigated and classified DenominatorTGT confirmed malaria cases present SourceMalaria case investigation database Target typeNon cumulative (C) Disaggregation of reported resultsReferencesDisease surveillance for malaria elimination- an operational manual, WHO, June 2013 |
| VC-3 (M) | GF - LLINs distributed continously |
DescriptionSpecify in the comments column of the performance framework which group(s) are being targeted NumeratorLong-lasting insecticidal nets distributed to targeted risk groups - through continuous distribution DenominatorNot applicable SourceProgram records of ITN distribution at specific sites Target typeNon cumulative (A) Disaggregation of reported results
|
| M&E-2 | GF - Facility reporting rate |
DescriptionFacility reports received over the reports expected NumeratorFacility reports received DenominatorFacility reports expected SourceNumerator: HMIS, program records Denominator: HMIS, program records Target typeNon cumulative (B) Disaggregation of reported results |
| M&E-2b | GF - TGT Facility reporting rate |
DescriptionTGT Facility reports received over the reports expected NumeratorTGT Facility reports received DenominatorTGT Facility reports expected SourceNumerator: HMIS, program records Denominator: HMIS, program records Target typeNon cumulative (B) Disaggregation of reported results |
4.2 Frequency of Reporting
4.2.1 HIV/TB
| Monthly | Quarterly | Semi-annually | Annually | Every two years | Continuously | Continuously – sentinel surveillance |
|---|---|---|---|---|---|---|
| M&E-1 | TCP-3 | TCP 6a | TCP 6a | HIV O-4a (M) | KP-1a (M) | TCS-3.1 |
| TCP-5 | TCP 6b | TCP 6bHIV I-6 | HIV O-5 (M) | KP-3a (M) | ||
| MDR-TB 4 | MDR-TB 2 (M) | HIV O-1(M) | KP-1c (M) | |||
| TCP-2 (M) | MDR-TB 3 (M) | TB O-1a | KP-3c (M) | |||
| TB O-4 (M) | Kp-1e | |||||
| TB I-2 | KP-3e | |||||
| PMTCT-1 | ||||||
| PMTCT-2.1 | ||||||
| TCS-1 (M) | ||||||
| TB/HIV-3.1 | ||||||
| TB/HIV-4.1 | ||||||
| TB/HIV-6 (M) |
4.2.2 Malaria
| Monthly | Quarterly | Semi-Annually | Annually |
|---|---|---|---|
| CM-1a(M) | |||
| CM-1c (M) | |||
| CM-2a (M) | |||
| CM-2b (M) | |||
| CM-2c (M) | |||
| CM-5 (M) | |||
| CM-6 (M) | |||
| VC-3 (M) | |||
| M&E-2 |